Neutron scattering
Atomic structure research of hydrogen storage materials with an advanced neutron diffractometer
Scope
On hydrogen storage process, atomic arrangement (atomic structure) tends to be changed, and in some cases, drastic structural changes such as crystalline structure to disordered atomic structure may happen.
It is essential to reveal these structural changes and the position of atoms for fundamental understanding of hydrogen storage mechanism in atomic scale.
Neutron scattering is quite powerful technique for such investigation, especially for positions of hydrogen atoms.
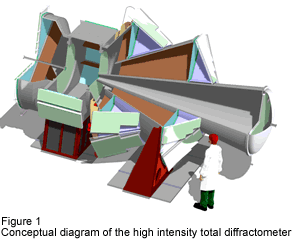 This group will install a high intensity total diffractometer (Fig.1) for hydrogen storage materials at pulsed neutron source of J-PARC, which is constructing at Tokai-village, Ibaraki prefecture (Fig.2). By measuring scattering of neutrons by samples (hydrogen storage materials) with this instrument, atomic structure changes during hydrogen storage will be investigated. With the highest level of neutron flux of J-PARC and state-of-the-art instrument devices and computing software, advanced research on hydrogen storage materials will be took place.
This group will install a high intensity total diffractometer (Fig.1) for hydrogen storage materials at pulsed neutron source of J-PARC, which is constructing at Tokai-village, Ibaraki prefecture (Fig.2). By measuring scattering of neutrons by samples (hydrogen storage materials) with this instrument, atomic structure changes during hydrogen storage will be investigated. With the highest level of neutron flux of J-PARC and state-of-the-art instrument devices and computing software, advanced research on hydrogen storage materials will be took place.
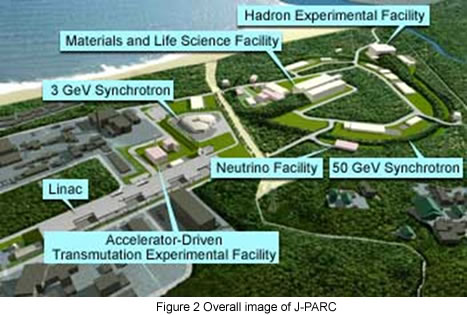
Figure 2 shows an example of atomic structure model obtained from data of total diffractometer at KEK.
In both figure, small red balls are hydrogen (deuterium). Left figure is the case of iron-terbium amorphous alloy.
"Amorphous alloy" is a solid in which there is no long-range order of the positions of the atoms.
Iron atoms (blue balls) and terbium atoms (yellow balls) are spatially separated.
On the other hand, in nickel-terbium amorphous alloy, nickel and terbium atoms are homogeneously mixed where nickel atoms are blue and terbium atoms are yellow.
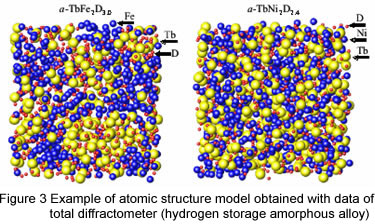
Such separation of atoms suggests higher endurance of nickel-terbium amorphous alloy.
This structural difference is caused by different affinity hydrogen for iron and nickel.